Generation and investigation of ultrafast phenomena with X-ray free-electron lasers
X-ray free-electron lasers (XFELs) deliver intense light pulses with femtosecond temporal resolution and hundreds of nanometers spatial resolution, thus making them an ideal tool to probe the molecular-scale structure of matter on timescales that are characteristic to molecular motion—or, in other words, to record "movies" of atoms and molecules moving and reorganizing into different structures. However, since XFEL facilities are a very recent development in the field of X-ray science, new techniques must be invented to study of a given phenomenon.
Our scientific interests reside at the intersection of XFELs with hydrodynamics, metastable systems, and phase transitions. To conduct such experiments, we developed new time-resolved investigation techniques. A major part of our recent work focuses on hydrodynamic phenomena induced by X-ray lasers in liquids. We are also involved in collaborative studies of rapid ice crystallization in water, of the chemical dynamics during the photosynthetic cycle in nature, and of the dynamics of biomolecules under the extreme conditions generated by XFEL irradiation.
A note: what is ultrafast? We use "ultrafast" here in the broad sense of processes that are currently too "fast" to be investigated experimentally. Thus, "ultrafast" depends on the system being studied, and can range from attoseconds to milliseconds. XFELs can open up new science at all these time scales.
Liquid explosions induced by XFELs
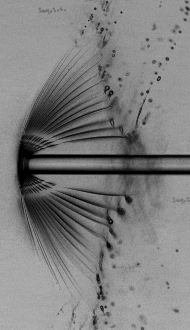
X-ray pulses produced at XFEL facilities are so intense that a single one is sufficient to destroy the sample under investigation. Often, XFEL experiments must be performed serially on a large number of identical samples, with a new sample for each X-ray pulse. The most frequently used technique for enabling such serial experiments is embedding the samples in continuously flowing liquid jets. The jets are also subject to X-ray damage, which may impede efforts to run experiments at high repetition rates.
We used time-resolved, high-resolution optical imaging to determine the dynamics of the explosions induced by XFEL pulses in liquid microjets. Our initial goal was to determine the maximum extent, in time and space, of the damage. We found that the effects of explosions can still be seen at time delays a billion times longer than the duration of the pulse, and affect the jets over distances up to a hundred times larger than the diameter of the X-ray beam. The XFEL explosions are multistep processes in which rapid high-energy phenomena drive slower dynamics. For example, picosecond isochoric heating by X-rays drives nanosecond shock waves, which in turn lead to spectacular microsecond hydrodynamics.
Serial crystallography at MHz repetition rate XFELs
A second generation of XFEL facilities is just starting to become operational (EuXFEL in Germany and LCLS-II in California). Their most important feature is that they can deliver hundreds to thousands more pulses per second than previous facilities – thus allowing much faster data collection and making these facilities available to many more researchers than currently possible. However, the very short interval between pulses, down to a fraction of a microsecond), leads to physical processes that may damage the samples and must be avoided.
We participated in some of the very first experiments at EuXFEL, which focused on recording protein crystallography data at very high (MHz) pulse rates. We used our expertise in understanding XFEL-induced explosions and shocks to diagnose their negative effects on samples, and develop data collection procedures that avoid these effects.
We have proposed that a side effect of running these experiments at very high pulse rates is damage the crystals due to the shock waves induced by the previous X-ray pulses. This effect is expected to occur only when the MHz XFELs are run at their highest pulse rates, which were not available when the first MHz XFEL opened in Germany. Therefore, we designed a special setup at the LCLS facility in California to simulate the effect of very high pulse rates, using nanosecond-spaced pairs of pulses, and we observed that protein crystals are affected by the shocks if the shock pressure exceeds a certain threshold.
Generation of deeply-metastable stretched water with XFELs
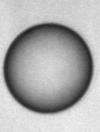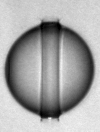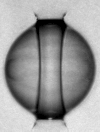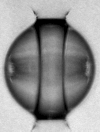
If a liquid with free interfaces is at a pressure below its vapor pressure, it is not thermodynamically stable and will either evaporate or boil. Nevertheless, it is possible to generate (metastable) liquids at pressures below the vapor pressure, or even in stretched states (corresponding to a negative pressure). Liquid water can be subjected to negative pressures for extended periods of time, but will cavitate if stretched too far. Previous experimental work dedicated at producing stretched water led to proven techniques for producing metastable water at pressures down to about −300 atmospheres, but this pressure is far from the pressure expected for homogenous cavitation (around -1500 atmospheres), implying that cavitation in pure water occurs though a heterogeneous cavitation mechanism that is specific to water.
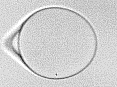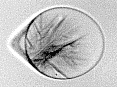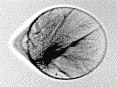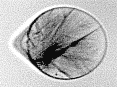
Using the reflection of shock waves produced by XFEL pulses at the surface of drops of water, we stretched water until it ruptured through spallation, on a few-nanosecond time scale. We determined from the spallation dynamics that pressures below -1000 atmospheres were briefly reached in the liquid—deeper than in all previous experiments that used unconfined water. We proposed a heterogeneous cavitation model that predicts that in order to break the apparent “negative pressure barrier” of -300 atmospheres, water must be stretched in a few nanoseconds or less. While such stretching rates may appear impractically rapid for investigations, there are ultrafast optical and X-ray techniques that can easily probe nanosecond processes, and application of these tachniques may be the key to studying water under previously inaccessible metastable conditions.
Metastable ice
Normal water ice, such as the ice we make in our freezers, has a hexagonal molecular symmetry ("hexagonal ice"), which manifests itself in the familiar hexagonal shapes of showflakes. But with a slight change in how the molecules are arranged in ice, crystals with cubic crystal symmetry ("cubic ice") may form. Observation of haloes around the sun suggest that crystals of pure cubic ice may form in the atmosphere, and if this is the case, its difference in vapor pressure relative to normal ice will affect how rain and snow form in the clouds. Scientists have observed that the freezing of supercooled water in the lab produces metastable ice, and this ice is neither perfectly cubic or hexagonal, but a hybrid structure with stacking defects.
A topic of interest in the field is how much "cubic-like" is the ice produced by freezing, and previous data indicated that the ice is more cubic when the water is more supercooled before freezing. We collaborated with Barbara Wyslouzil's group from Ohio State to record X-ray diffraction from nanosized water droplets frozen after being supercooled to below -50 °C. We colected data less than 100 microseconds after freezing, and our analysis indicated that our ice was 78% cubic, more than other ices produced by freezing by supercooled water. After this, other researchers made pure cubic ice, although by different means than simply freezing supercooled water.
Droplets in clouds are micron-sized, less supercooled than the nanodrops, and their freezing is a complex process. To understand the first stages of freezing, we collected not only X-ray diffraction but also many optical images of supercooled 40-µm drops freezing in a vacuum chamber. We developed a seven-stage model of freezing at the scale of the drop, and we also used the model to "time" the X-ray diffraction data. We observed that there is a fast but gradual development of long-range order in the crystal, which produces a type of metastable ice not observed before. While it is very likely that this type of ice is short-lived and transforms to other types of metastable ice, observing it is important for unerstanding the molecular mechanism of rapid nonequilibrium freezing.